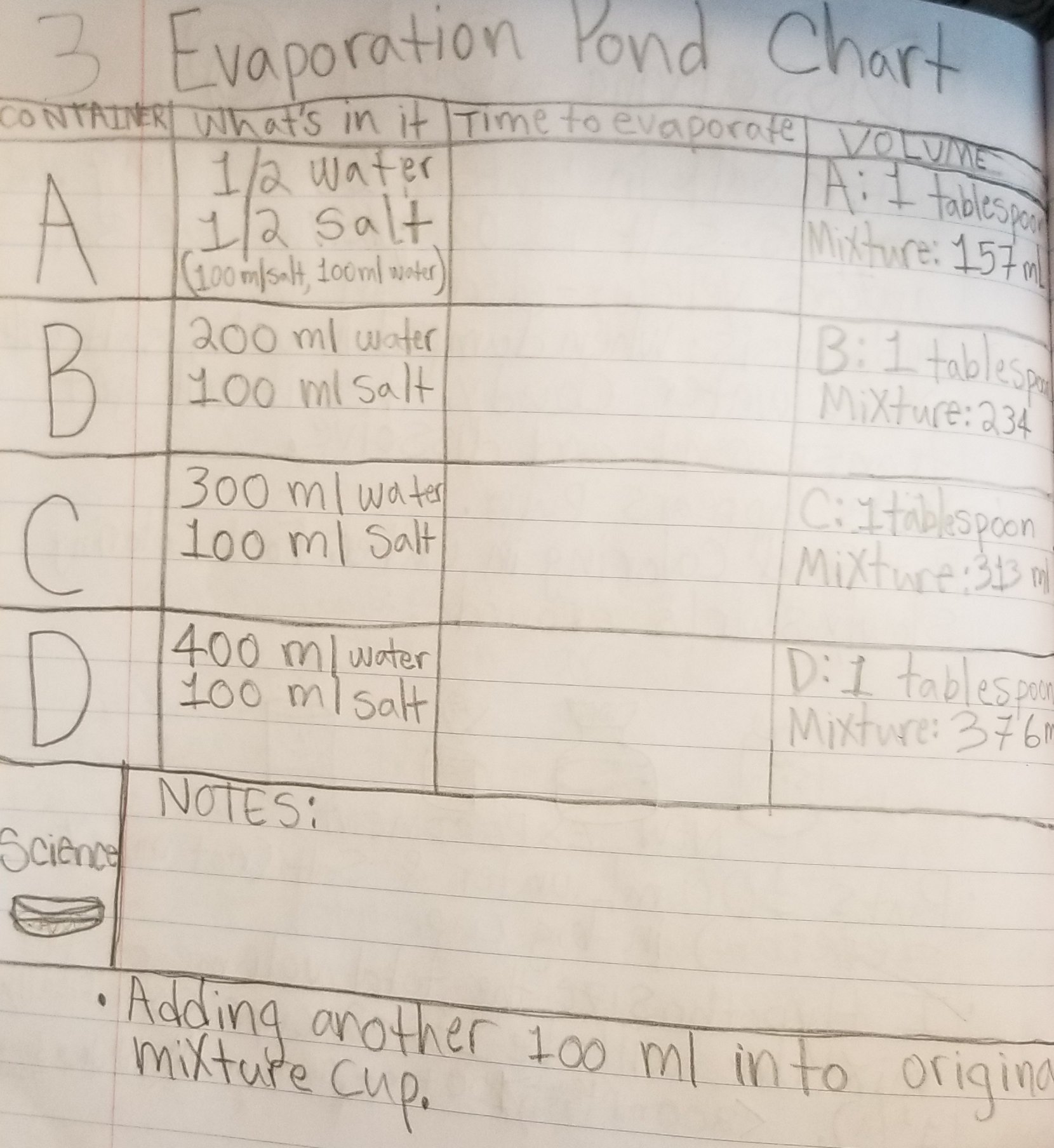
Last week we did an experiment in which we made four solutions with varying concentrations of salt (1:1, 1:2, 1:3 & 1:4 salt to water ratio). We let them evaporate and took a look at the result in class today. 
Everyone agreed that the result in the 1:4 ratio was pretty cool. But why the cool crystals? Was it an anomaly? (New vocabulary word 🙂 ) The students immediately began hypothesizing that it must be connected to the different concentrations. But to be sure, we would need to do what scientists do — do another trial and see if the same thing happens. We started that, and also began thinking of theories that would explain what we saw.
In this 2nd trial, we added a few additional concentrations ranging from 1:1 to 1:6 to see if the pattern would continue that bigger crystals form in solutions with lower concentrations of salt.
It’s very satisfying to watch the group intuitively deriving the scientific process, rather than me having to do a boring lecture about the steps of a science fair project!
Homework this week is to read pages 16 to 19 (FOSS Gr 5) and look for clues that would explain the crystal formation we saw. It’s all there, but it might take a little support from a parent to make the connection. Please don’t just give them the answer, but help guide them. Here’s your cheat sheet:
A supersaturated solution tries to hold onto its dissolved material until that dissolved material finds something it can begin to crystallize around. As the numbers in the book show, the first 3 solutions have more salt than can be dissolved in water, and therefore there are hundreds of salt crystals on the bottom of the trays. As the water begins to evaporate, the salt in the water grows around these tiny salt crystals. You can see this in the leftmost three trays above. But in the rightmost three trays with a concentration of 1:4 or less, there are no salt crystals to seed crystal formation – so there’s nowhere for the salt to go. Eventually, the crystal must form — perhaps around a dust particle? — when most of the water is gone. When that first crystal forms, all the nearby salt frantically forms a big crystal on it. And that’s why we see a few big crystals.
After learning that the term “salt” refers to a more general chemical concept, we jumped into playing with oobleck — a cool combination of cornstarch and water that has very unusual properties.

Again, we tried to guess why this might be the case. We looked at oobleck under a microscope and saw how its shape and buoyancy could explain what was going on. We also looked at this image from a fancier microscope to get a better view.

At that point, everyone was a mess… Covered in oobleck. So we finished class playing with it outside!